Dive Brief:
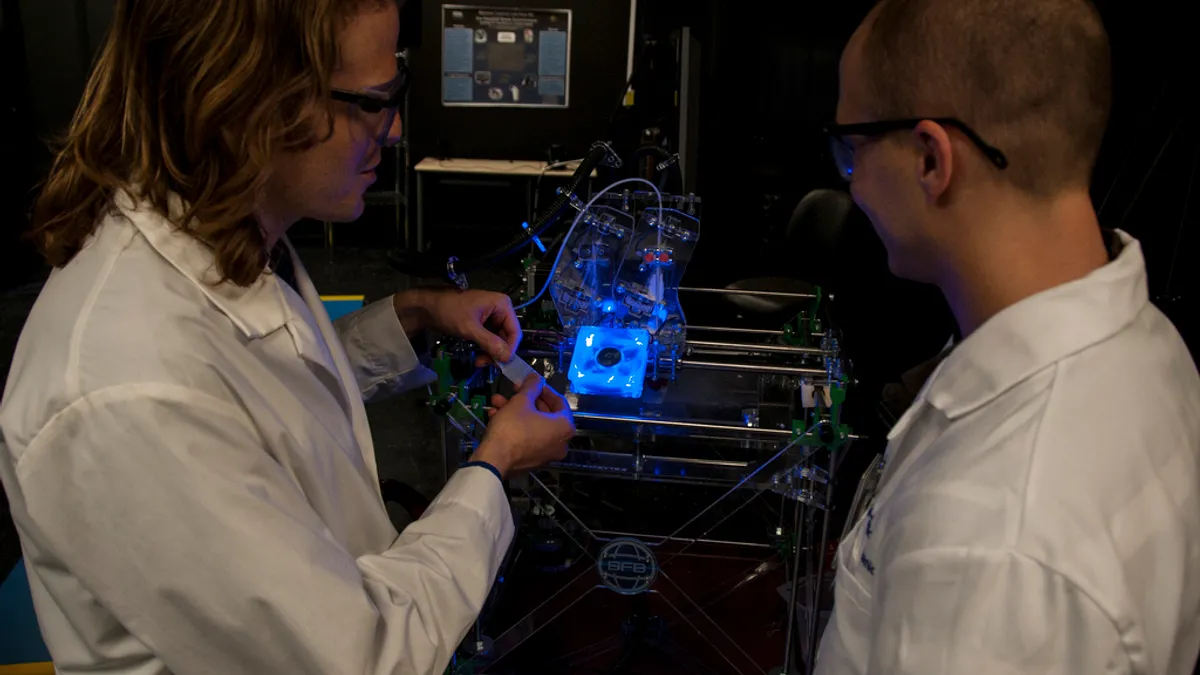
- The Food and Drug Administration's recent approval of a 3-D printed drug opens a world of possibilities for the pharmaceutical supply chain, which has traditionally depended on far-away factories for production, PharmaBiz reports.
- Rather than producing to economies of scale, 3-D printing at point of demand means medication in table form can be created much closer to the patient, and could allow doctors to improve treatment by creating tailored dosing regimes for patients.
- In addition, 3-D printing could facilitate the creation of drug release profiles and may, in as early as 20 years, allow for the development of living tissue, according to The Next Web.
Dive Insight:
Thus far, only Aprecia, an Ohio based drug maker, has harnessed the capabilities of 3-D printing to personalize one of its epilepsy medications.
The technology for such patient-centric modifications is hardly around the corner, though. While the FDA's approval of Aprecia's drug shows the regulator is open to 3-D applications for pharmaceuticals, significant R&D investments must be poured in to show the safety applications of the drug.
As a result, if the technology gains ground in the pharmaceutical sector, it will first affect long-established drugs, for which new methods of production are unlikely to pass a cost-benefit analysis. Rather than pharmaceuticals, then, the technology has implications for healthcare, wherein 3-D printing is already gaining ground through. The Next Web reports 21% of healthcare investments will go to 3-D printing over the next decade.
Regardless, the examples show 3-D printing technology is here to stay, and could have implications on the locations and inventory needs throughout the medical supply chain.