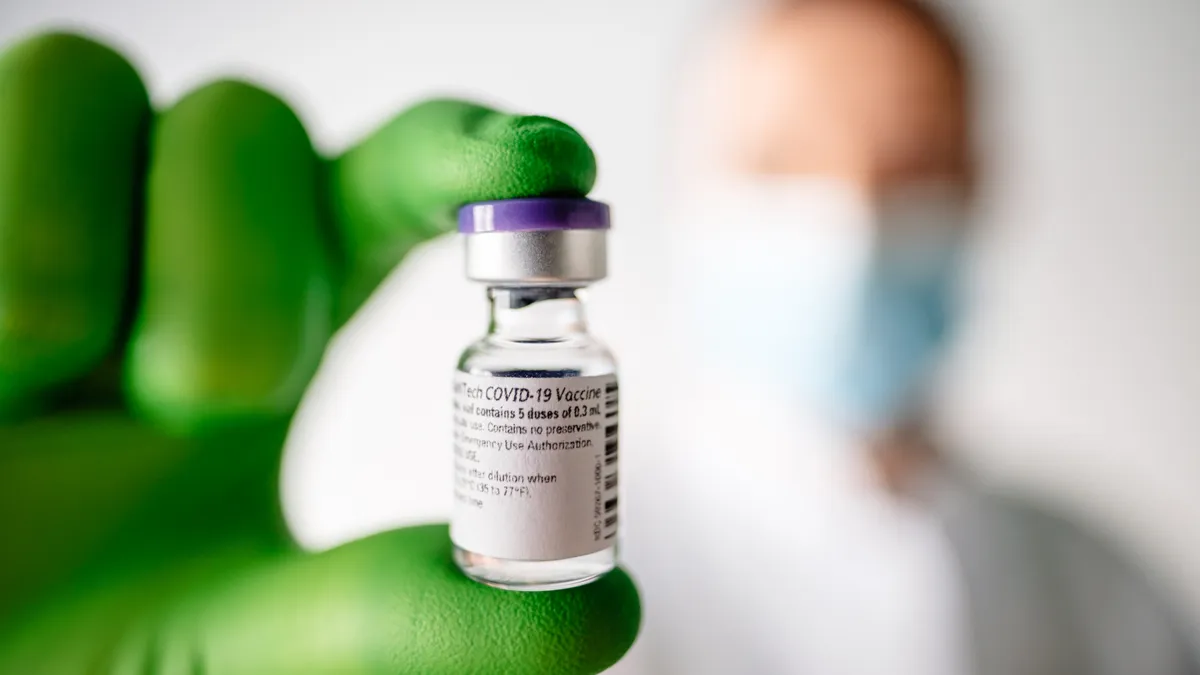
The vaccine supply chain is ready to start delivery to inoculation sites after multiple tests in recent weeks and a planning effort that began this summer, according to Operation Warp Speed Chief of Staff Col. Eric Shirley.
"OWS is ready to begin distribution of those vaccines within 24 hours of that [emergency use] authorization," Shirley said Tuesday in an interview with Supply Chain Dive.
The EUA arrived late Friday from the Food and Drug Administration, clearing the way for distribution of Pfizer's and BioNTech's coronavirus vaccine.
The authorization followed a Thursday meeting that concluded with a group of independent advisers to the FDA backing use of the vaccine.
"This is just the first step as the federal and state governments partner with the healthcare supply chain to mobilize a logistics undertaking unlike any this country has ever seen," Chester Davis Jr., CEO of the Healthcare Distribution Alliance, said in a statement after the authorization.
If the U.S. is going to meet its goal of vaccinating the nation by the middle of 2021, it will require 16 million package deliveries to thousands of vaccination sites within 64 jurisdictions. It's an effort that Shirley acknowledged "sounds daunting."
A series of distribution tests
OWS began to hold working groups in the summer with distribution partners such as AmerisourceBergen, Cardinal Health and McKesson, Shirley said.
The summer meetings helped the government to understand the companies' capacity and distribution networks. The Centers for Disease Control and Prevention provided a contract to McKesson for vaccine distribution following the meetings.

Since September, OWS has held weekly table-top exercises to help practice the distribution of the vaccine with partners including McKesson, FedEx and UPS.
The distribution partners are also part of the daily operational calls, Shirley said.
"When the EUA comes, no one is having to reach out and have coordination meetings," he said in an interview before the FDA's authorization. "By that point, it will be a very scripted, very highly refined distribution plan that takes effect."
In recent weeks, OWS has taken this testing from the table-top to the real world, with end-to-end pilot distribution to test the physical and digital delivery infrastructure. An initial test run with Pfizer included 10 jurisdictions, which was expanded to more locations in another test last week. The tests have included assessments of Pfizer's custom-made shipping boxes.
"As we continue to look at the stability data for Pfizer and they have continued to work on the distribution and packaging for their product, what we're finding is that this cold chain requirement becomes entirely manageable," Shirley said.
Tracking delivery to inoculation sites
The jurisdictions have told OWS leaders the addresses of their ultra-cold storage locations. The OWS technology infrastructure will let the leaders know when a location receives a vaccine delivery, and a dry-ice delivery will automatically be set for 30 days from that point.
"When you think about the volume ... amount of actual product that's going out, think about what happens each and every day with UPS and FedEx deliveries," Shirley said. "It's a very important delivery. But it is no more complex than taking physical distribution street addresses, confirming that there is a valid order into the VTrckS system, and delivering."
VTrckS is the Vaccine Tracking System that allows the CDC to track the distribution of publicly-funded vaccines, according to the agency.
State health officials have also been planning for the roll out of the vaccine since the summer, according to Virginia Department of Health Director of the Division of Immunization Christy Gray. Virginia set up its vaccine advisory workgroup in September, Gray said.
Part of the state-level planning was determining what locations had the freezer capacity to accept the vaccines and, in the case of Pfizer's vaccine, ultra-cold freezers. In Virginia, VDH worked with the Hospital and Healthcare Association to identify the locations. Equipment is being added to the state network daily, Gray said, noting that the state is working to ensure equitable access to the vaccine.
"It will be a very scripted, very highly refined distribution plan that takes effect."

Col. Eric Shirley
Chief of Staff for Operation Warp Speed
Gray said Virginia worked with McKesson on distribution for two of its vaccine programs and has developed a strong relationship. The process for the COVID-19 vaccine is more of the same.
"We're using the same ordering system that we used for those programs," she said in an interview with Supply Chain Dive. "So this is just ... another type of vaccine that we are working to the same process we are already very comfortable doing and very experienced doing."
Virginia will place its order in VTrckS, where it can see the number of doses available to the state. The state will then assign the doses to immunization locations and place the order with either McKesson or Pfizer. This triggers a delivery of ancillary supplies to be delivered to the same location to arrive before or at the same time as the vaccine.
Shirley said UPS and FedEx will provide white glove delivery of the vaccine to the front door of the recipient location.
"Separate and distinct from their normal bulk deliveries of bandages and other resupply, these vaccine deliveries to our pharmacy partners are going for guaranteed delivery to those locations," he said.

OWS will track the deliveries from their departure until they get to the administration site. It is using a data platform called Tiberius to help visualize data from multiple sources to monitor the operation. Tiberius brings together data from various agencies and includes information on manufacturing, supply chain, allocation, state-level planning and other information. It will also be used to calculate allocations for the initial distribution, according to the Pentagon's website.
"The jurisdictions will work inside the Tiberius platform to decide where every allocated dose will go — from local doctors' offices to large medical centers," the agency explained. "These decisions will then be sent to distributors to complete deliveries across the country."